A number of physical properties can be calculated from an equation of state combined with a model for an ideal gas heat capacity.
VLXE output the most common:
•Enthalpy
•Entropy
The two most common expressions for the ideal gas heat capacity in VLXE are:
•Polynomial
•DIPPR
The user sets the model independent for solvents and polymers.
Reference temperature and pressure
When calculating e.g. enthalpy and entropy a reference temperature and pressure is needed in the integration of the ideal gas expression,
The values used in VLXE are:
Reference temperature: 298.15 Kelvin
Reference pressure: 1.0 bar
Polynomial
The polynomial is given as:
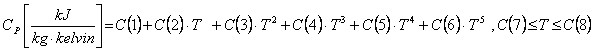
Note the units of Cp.
C(7) and C(8) defines the temperature range where the expression is valid
For co-polymer the formula used is:
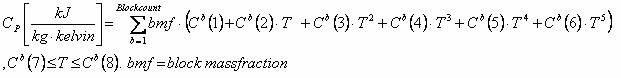
DIPPR
The DIPPR expression is given by:
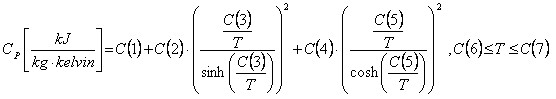
Note the units of Cp.
C(7) and C(8) defines the temperature range where the expression is valid
For co-polymers the formula used is:
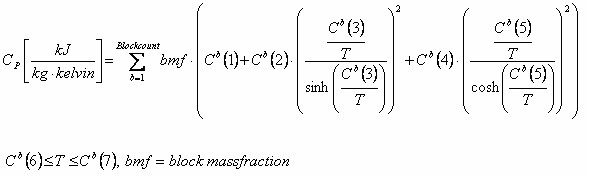
Often the parameters used are reported as dimensionless values,however it is converted into units as:
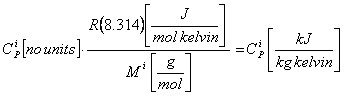
Note that in the DIPPR expression only C1, C2 and C4 has to be converted.
Poly disperse polymers
Polymers that are poly disperse poses a special problem since the parameters for the ideal heat capacity expression cannot be given for each pseudo component and often is not known.
The method that is used in VLXE is based on the fact that for heavy alkane's the value for Cp on a mass basis becomes independent on the alkane as the molar mass becomes big.
This information is used, so only one set of parameters has to be given for a polymer. The parameters are then scaled using the molar mass of each pseudo component.
Parameters for a given polymer mono-/poly-disperse
If no values are available for a given polymer it is recommended to use the values for a C20H42: Eicosane.
The values are:
C(1) |
C(2) |
C(3) |
C(4) |
C(5) |
C(6) |
C(7) |
C(8) |
|
Polynomial Cp(kJ/kg K) |
8.1694E-1 |
-3.0569E-4 |
1.5706E-5 |
-2.1058E-8 |
8.5058E-12 |
0 |
200 |
1000 |
DIPPR Cp (kJ/(kg K) |
1.1496 |
3.9249 |
1636.0 |
2.6367 |
-726.27 |
200 |
1500 |
- |
Not much research has been done in this field. As more research is done VLXE will become updated.
Please contact VLXE if you prefer another method to included in.